 |
Liquid metal "ultra-low temperature" extracted silicon
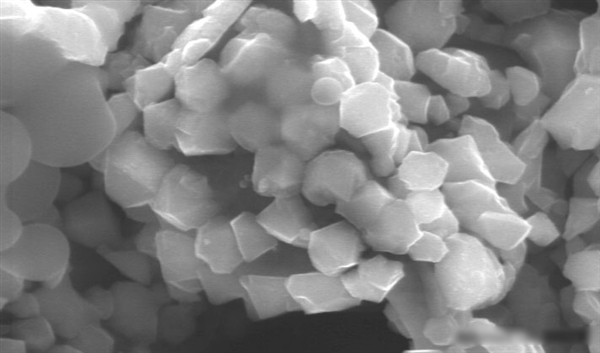
Electron micrographs of pure silicon crystals extracted from liquid metals
Silicon is the backbone of modern electronics, but its production has long been a costly and environmentally damaging process. Traditionally, it's extracted from sand through high-temperature chemical reactions that require extreme heat—over 2000°F (1350°C)—and produce large amounts of carbon dioxide.
Now, researchers at the University of Michigan have developed a groundbreaking method to extract crystalline silicon at just 180°F (82°C), which is roughly the internal temperature of a fully cooked turkey. This low-temperature approach could revolutionize the semiconductor and solar energy industries.
The team, led by Professor Stephen Maldonado, used liquid metal as a medium instead of water, and silicon tetrachloride instead of sugar. Just like how sugar crystallizes in a supersaturated solution, their process allows silicon to form crystals within the liquid metal without the need for additional heat.
"We’re not using water, we’re using liquid metal," Maldonado explained. "And we’re not using sugar—we’re using silicon." By passing an electric current through the liquid metal, they were able to convert silicon tetrachloride into pure, crystalline silicon.
The key to the process is the use of liquid metals, which can provide electrons to facilitate the reaction without requiring extra heating. Solid-state methods may do similar things, but only liquid metals can enable crystallization in a liquid state.
While the current silicon crystals are very small—only about 0.5 microns in diameter—researchers believe this technology can be scaled up. They are exploring other low-melting alloys to improve crystal size and practical applications.
If successful, this method could drastically reduce the cost and environmental impact of producing silicon. It could make solar panels more affordable and open new possibilities in semiconductor manufacturing.
Maldonado admits it’s too early to predict exact cost savings, but he sees great potential. "This could be a flexible, cheap, and eco-friendly way to produce silicon," he said. "Our dream is to turn sand into crystalline silicon in one step. That’s not impossible anymore."
The University of Michigan is already working on patenting the technology and seeking industry partners to bring it to market. One day, this breakthrough might even be used in everyday devices like computer processors.
JIANGSU ARTSTYLE DECORATION MATERIALS CO..LTD , https://www.artstyledecor.com